Case Report - (2024) Volume 11, Issue 5
Complete penoscrotal transposition associated with anorectal malformation: A case report
Habou Oumarou1*, Hamissou Laouli Saadou1, Ali Ada Mahamoud Omid2, Moustapha Hellé2, Halidou Maazou3 and Abarchi Habibou2Abstract
Complete Penoscrotal Transposition (CPST) is an exceptional and complex urogenital malformation, of etiology not yet understood. We report the case of a new-born admitted to our department for external genitalia anomaly with anal imperforation. Physical examination reveals a complete penoscrotal trans position, with a normal scrotum and hypoplasia of the penis, associated with a totally occlusive anorectal malformation. At the invertogram, the rectal cul de sac was located in the Stephens-Kelly triangle. The malformation assessment did not objectify other anomalies. A colostomy was performed as an emergency and the correction of the anorectal malformation by Posterior Sagital Anorectoplasty (PSARP) was done at the age of 3 months, followed by closure of the stoma 3 weeks later. At 20 months, a surgical correction of de CPST by penis up transfer was performed. The recovery was uneventful and cosmetic result was satisfactory at 4-months follow-up.
Keywords
Penis, Anomily, Penoscrotal transposition, Surgery
Introduction
Complete Penoscrotal Transposition (CPST) is an exceptional and complex malformation, first described by Appleby in 1923 [1]. Its etiology remains unknown, but seems to result from abnormal development of the genital tubercles between the 5th and 7th week of gestation [2,3]. It is characterized by a mirror inversion of the external genitalia with a penis, usually hypoplastic, located caudally and posterior to the scrotum. CPST is most often sporadic, but familial cases have been reported [2,4]. There are 3 varieties: The form with normal scrotum, the form with scrotal bifidity and the form with abnormalities of the posterior pelvis. Given the rarity of reported cases, there is no unequivocal attitude for the correction of this anomaly. We report the case of new-born with complete penoscrotal transposition, with normal scrotum, associated with an anorectal malformation.
Case Presentation
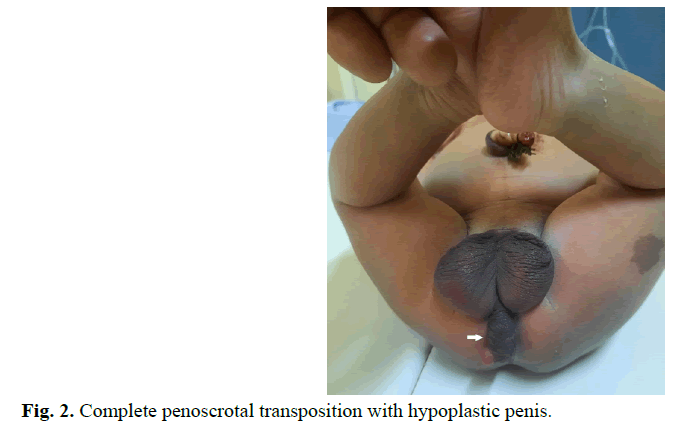
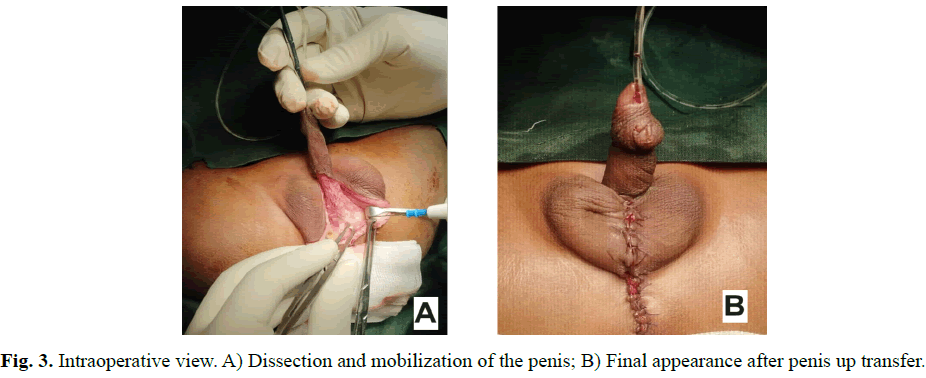
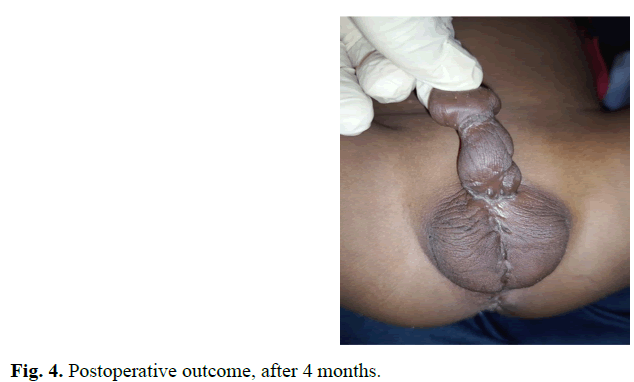
A male new-born on day 2nd of life is admitted to our department for malformation of the external genitalia and a lack of anal orifice. He was born full term to a non-consanguineous couple by caesarean section. No antenatal history was noted, the pregnancy was well monitored; obstetric ultrasounds did not reveal fetal abnormalities. At birth he had a good adaptation to extrauterine life, APGAR 10, weight at 3500 grams, height at 49 centimeters and head circumference at 33 centimeters. Physical examination revealed abdominal distension without collateral venous circulation, absence of the anal orifice with depression at the normal position of the anus, a slight intergluteal fold and posterior transposition of the penis in relation to the scrotum (Figures 1 and 2). Both testicles were palpable in the bursa. The penis was of normal size, with hypoplasia of the corpora cavernosum. No other external genital anomalies or clinically visible associated malformations were observed. X-Ray Abdominal invertogram revealed rectum located in the Stephens-Kelly triangle. The malformation assessment did not objectify other anomalies. The diagnosis of CPST associated with an intermediate Anorectal Malformation (ARM) was retained and a colostomy was done in emergency. ARM correction by PSARP was performed at three months of life and closure of stoma was done 3 weeks later. At 20 months the clinical assessment was satisfactory and surgical correction of de CPST, by penis up transfer, was performed (Figure 3). The recovery was uneventful and cosmetic result was satisfactory at 4-months follow-up (Figure 4).
Fig. 1. Absence of the anal orifice, with a depression in the normal position of the anus and a faint intergluteal fold (Black arrow).
Fig. 2. Complete penoscrotal transposition with hypoplastic penis.
Fig. 3. Intraoperative view. A) Dissection and mobilization of the penis; B) Final appearance after penis up transfer.
Fig. 4. Postoperative outcome, after 4 months.
Results and Discussion
Penoscrotal transposition is a rare urogenital anomaly. Its prevalence is estimated at 0.61 per 10000 live male births; complete form represents 26% of cases [5]. The etiology of penoscrotal transposition is not well understood. Apart from embryological disturbances (abnormal development of genital tubercles around the 6th week of gestation; abnormal location of the genital tubercle or delay in the median fusion of the labioscrotal folds) whose sequence is not well elucidated other etiological factors have been reported, including exposure to radiation or infectious factors [6-10]. In the majority of cases reported in the literature, the diagnosis of penoscrotal transposition was made postnatally. However, prenatal diagnosis of this anomaly is possible and can help for counselling the parents and preparing for postnatal care [10].
Rarely isolated, CPST is most often associated with other urogenital abnormalities (hypospadias, testicular migration anomaly, urethral atresia, penile hypoplasia, etc.) [5,11-13]. This common association of urogenital anomalies would suggest that these anomalies share or have intricate mechanisms in their etiologies [5]. Other less frequent abnormalities, associated with TPSC, have been reported (gastrointestinal, cardiac, orthopedic, etc.) [6,7,14]. In our case, it is associated with a totally occlusive anorectal malformation that had required an emergency procedure by performing a right transverse colostomy.
The genetic study of penoscrotal transposition shows a diversity of chromosomal lesions associated with this malformation of all these lesions, only mosaic trisomy 18 is associated with complete penoscrotal transposition [10]. The 13q22 deletion is frequently reported in the literature as a genetic factor associated with numerous anogenital anomalies. This suggests the role of chromosome 13 in the embryological development of these structures [5,15-17]. Penoscrotal transposition has been mentioned in some X-linked syndromes (Klinefelter syndrome, Simpson-Golabi- Behmel syndrome, etc.) [9,18,19]. These discoveries suggest that a karyotype should be performed in any patient with penoscrotal transposition in search of chromosomal abnormalities in our case, due to a lack of technical facilities and the financial resources of the parents, this examination could not be carried out [10].
Management of CPST is a challenge in pediatric urology. Indeed, given the rarity of cases reported in the literature, there is no unequivocal technique for the surgical correction of this anomaly. This surgery can be done in one or several stages and is performed between 12 and 18 months [3,8,11,12]. We performed the penile transposition upwards in a time of 22 months. According to Wu et al., this method is easier to perform and gives fewer postoperative complications than the scrotal flap method [20]. Overall, the result of this surgery must take into account the presence of other associated urological abnormalities such as severe hypoplasia of the penis or hypospadias with significant elbowing. In our case, the cosmetic result is satisfactory but given the hindsight, we cannot comment on the genital prognosis.
Conclusion
Surgical correction of CPST is difficult and has a dual purpose, aesthetic and functional (urinary and genital). Several techniques have been proposed, but the rarity of reported cases makes it difficult to compare these different procedures. The choice of technique should take into account the severity of the malformation and associated abnormalities, as well as the surgeon’s resources and skills.
Author Consent
Informed and written consent was obtained from the family.
Acknowledgments
We thank Dr. Jun Ho for his contributions to this article.
References
- Appleby LH. An unusual arrangement of the external genitalia. Can Med Assoc J. 1923;13(7):514.
[Google Scholar] [PubMed]
- Naren SD, Soren C, Subbarao PV. Penoscrotal transposition: A case report. Indian J Surg. 2013;75(1):64-65.
[Crossref] [Google Scholar] [PubMed]
- Jourdainne VB, Peycelon M, Peev S, et al. One-stage surgery for complete penoscrotal detransposition: Technique and long-term evolution. Prog En Urol. 2020;30(13):853.
- Chung JL, Choi JR, Park MS, et al. A case of del(13)(q22) with multiple major congenital anomalies, imperforate anus and penoscrotal transposition. Yonsei Med J. 2001;42(5):558-562.
[Crossref] [Google Scholar] [PubMed]
- Allred RP, Nguyen J, Agopian AJ, et al. An epidemiologic study of penoscrotal transposition by maternal characteristics using data from the Texas birth defects registry. Birth Defects Res. 2024;116(1):e2270.
[Crossref] [Google Scholar] [PubMed]
- Pinke LA, Rathbun SR, Husmann DA, et al. Penoscrotal transposition: Review of 53 patients. J Urol. 2001;166(5):1865-1868.
[Crossref] [Google Scholar] [PubMed]
- Chadha R, Mann V, Sharma A, et al. Complete penoscrotal transposition and associated malformations. Pediatr Surg Int. 1999;15(7):505-507.
- Somoza I, Palacios MG, Mendez R, et al. Complete penoscrotal transposition: A three-stage procedure. Indian J Urol. 2012;28(4):450-452.
[Crossref] [Google Scholar] [PubMed]
- Sexton P, Thomas JT, Petersen S, et al. Complete penoscrotal transposition: Case report and review of the literature. Fetal Diagn Ther. 2015;37(1):70-74.
[Crossref] [Google Scholar] [PubMed]
- Kurt D, Sivrikoz TS, Kalelioglu HI, et al. Prenatal diagnosis of complete penoscrotal transposition with normal scrotum: Two case reports and review of the literature. J Clin Ultrasound JCU. 2020;48(6):350-356.
[Crossref] [Google Scholar] [PubMed]
- Beyazit F, Pek E, Aylanç H. A rare case of complete penoscrotal transposition with hypospadias in a newborn. Turk J Obstet Gynecol. 2017;14(1):74-75.
[Crossref] [Google Scholar] [PubMed]
- Imamoglu Ç, Osman AA. Complete penoscrotal transposition: A case report. Abant Med J. 2020;9(1):56-58.
- Huang H, Liu X, Li Z, et al. Ectopic scrotum and penoscrotal transposition: Case report and literature review. Front Pediatr. 2023;11:1015384.
[Crossref] [Google Scholar] [PubMed]
- Ayamba AM, Maalman RSE, Donkor YO, et al. Complete penoscrotal transposition with other extragenital anomalies in a neonate delivered at term. Case Rep Urol. 2021;6676301.
[Crossref] [Google Scholar] [PubMed]
- Gershoni-Baruch R, Zekaria D. Deletion (13)(q22) with multiple congenital anomalies, hydranencephaly and penoscrotal transposition. Clin Dysmorphol. 1996;5(4):289-294.
[Google Scholar] [PubMed]
- Garcia NM, Allgood J, Santos LJ, et al. Deletion mapping of critical region for hypospadias, penoscrotal transposition and imperforate anus on human chromosome 13. J Pediatr Urol. 2006;2(4):233-42.
[Crossref] [Google Scholar] [PubMed]
- Kurt D, Sivrikoz TS, Kalelioglu HI, et al. Prenatal diagnosis of complete penoscrotal transposition with normal scrotum: Two case reports and review of the literature. J Clin Ultrasound JCU. 2020;48(6):350-356.
[Crossref] [Google Scholar] [PubMed]
- Kawakami Y, Sawano K, Shibata N, et al. Klinefelter syndrome with penoscrotal transposition and diphallia: A case study. Congenit Anom. 2023;63(4):125-126.
[Crossref] [Google Scholar] [PubMed]
- Chaves JJ, Guerrero A, Bonilla JC, et al. Complete penoscrotal transposition: A two case report and literature review. Repert Med Cir. 2017;26(2):98-103.
- Wu Y, Hu Y, Wang S, et al. Effectiveness of penis up transfer method for penoscrotal transposition in children. J Reparative Reconstr Surg. 2022;36(6):776-780.
[Crossref] [Google Scholar] [PubMed]
Author Info
Habou Oumarou1*, Hamissou Laouli Saadou1, Ali Ada Mahamoud Omid2, Moustapha Hellé2, Halidou Maazou3 and Abarchi Habibou22Department of Pediatric Surgery, National Hospital Amirou Boubacar Diallo of Niamey, Abdoul Moumouni, Niger
3Department of Urology, National Hospital of Zinder, Andre Salifou University, Zinder, Niger
Received: 21-Aug-2024, Manuscript No. PUCR-24-146033; , Pre QC No. PUCR-24-146033 (PQ); Editor assigned: 23-Aug-2024, Pre QC No. PUCR-24-146033 (PQ); Reviewed: 06-Sep-2024, QC No. PUCR-24-146033; Revised: 13-Sep-2024, Manuscript No. PUCR-24-146033 (R); Published: 20-Sep-2024, DOI: 10.14534/j-pucr.20222675670
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.