Case Reports - (2024) Volume 11, Issue 5
Surgical assessment in Persistent Mullerian Duct Syndrome (PMDS) with intra-abdominal testes: A case report
Germana Casaccia1*, Lorenzo Giacometti2 and Andrea Secco3Abstract
Background: In a male foetus at 8 weeks’ gestation, testicular sertoli cells produce Anti-Müllerian Hormone (AMH) also known as Müllerian Inhibiting Substance (MIS). It plays a rule in the migration of testes in the scrotum and leads to regression of the Mullerian ducts, which typically differentiate into Fallopian tubes, uterus and upper vagina in a female foetus. The Persistent Mullerian Duct Syndrome (PMDS), due to AMH or AMH receptor defect, is a rare condition characterized by cryptorchidism and rudimentary internal female genitalia in otherwise normally masculinized 46 XY male. Herein the author reports a pediatric case of PMDS with bilateral intra-abdominal testes, focusing on the correct diagnosis and a rational stepwise surgical treatment. At last follow up, the twelve years old patient presents bilateral healthy scrotal testes with no Mullerian ducts remnants and hormonal dysfunction.
Keywords
Persistent mullerian duct syndrome, Bilateral cryptorchidism, Orchidopexy
Introduction
The Persistent Mullerian Duct Syndrome (PMDS) typically presents with three clinical patterns: Bilateral cryptorchidism in more than half of the patients, unilateral cryptorchidism with contralateral hernia in 20% of them, and Transverse Testicular Ectopia (TTE) in the rest. Abnormalities of male excretory duct are frequent and particularly associated with intra- abdominal cryptorchidism [1]. Unilateral or bilateral cryptorchidism is not mainly evocative of syndrome, although unilateral cryptorchidism with contralateral hernia and particularly Transverse Testicular Ectopia (TTE) should awake suspicion. Bilateral cryptorchidism is a challenging congenital anomaly to handle appropriately already in an otherwise healthy patient. In PMDS, it requires a further skill and learning. An instructive PMDS pediatric case with intra-abdominal testes is reported.
Case Presentation
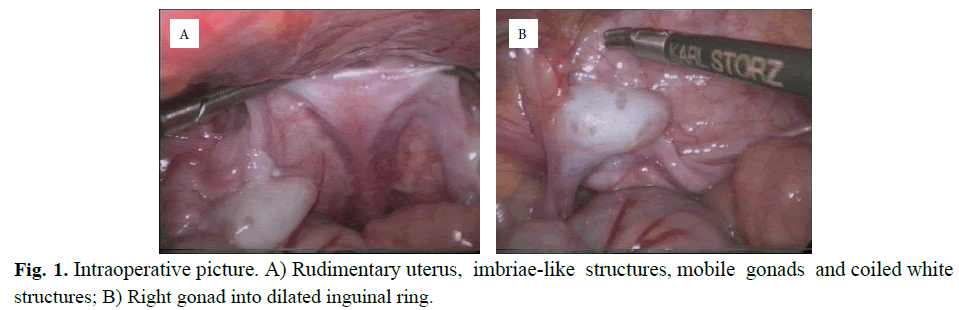
At 10 months of life, the patient was referred to our clinic due to non-palpable bilateral testes. He presented outwardly completely male; the external genitalia were not ambiguous with the urethra opens at the tip of the penis and a good scrotal fold on both sides. The ultrasound scan confirmed the absence of testes in the groin and scrotum bilaterally. At 14 months a laparoscopy was performed. During the procedure the presence of a rudimentary uterus in the center of pelvis and fallopian tubes with fimbriae like structure directly above the gonads, were observed. No macroscopic normal epididymal structures were recognized. At each side, next to the rudimental uterus walls, white and coiled structures were identified. Apparently the gonads presented a high degree of mobility reaching easily the corresponding contralateral internal ring. Both inguinal internal rings showed to be widely opened (Figure 1).
Fig. 1. Intraoperative picture. A) Rudimentary uterus, imbriae-like structures, mobile gonads and coiled white structures; B) Right gonad into dilated inguinal ring.
Upon this appearance confused the surgeon regarding the real genetic and gonadal nature of patient, so a formal right inguinal hernia repair associated with an orchidopexy and a gonadal biopsy were performed at first stage. Afterwards the patient was studied to perform a correct diagnosis. The serum Anti-Mullerian Hormone (AMH) levels were undetectable. At genetic study, a normal male 46 XY karyotype and two different mutations of the AMH gene were identified: c.350_351insCTGGGGGCCTGGCTGCGGGACC (p.Leu118Trpfs*63) in the first exon and c.1669T>G (p.Cys557Gly) in the fifth exon. The mother agreed to undergo to the genetic examination and she was recognized to be heterozygous for the mutation of the first exon of AMH gene. The biopsy reveals normal testicular morphology (Figure 2).
Fig. 2. Histological pattern of testicular biopsy.
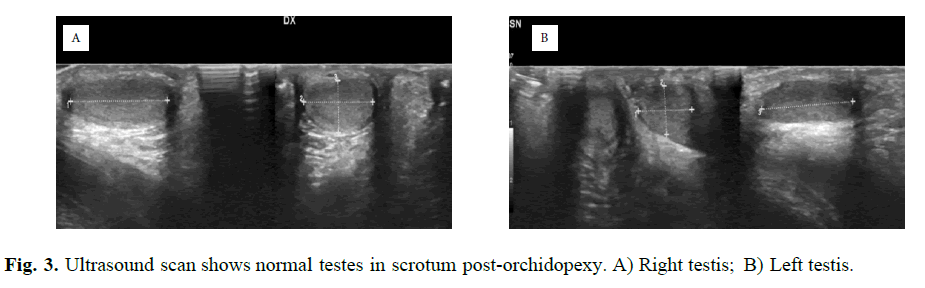
On the basis of these data, a PMDS was diagnosticated. At follow up, the right testis was find healthy in the upper scrotum, still covered by a fimbria like structure, after three months. At 17 months, formal left inguinal hernia repair with orchidopexy was performed. The testis flipped to the groin by pulling the sac and before to bring it down in the scrotum, it was freed from both the fimbriae like structure and the sac. The right testis necessitated another inguinal approach orchidopexy procedure to remove the same structure that avoid it reaching correctly the scrotum. An ultrasound scan after six months revealed normal testes in the scrotum, no epididymal structures were recognized (Figure 3).
Fig. 3. Ultrasound scan shows normal testes in scrotum post-orchidopexy. A) Right testis; B) Left testis. A B
At 5 years, a third stage laparoscopic procedure was performed to resect intra-abdominal Müllerian remnants. These structures were easily isolated and removed. A rudimental uterus entered the upper urethra at the level of the prostate, with apparently obliterated communication. Concomitantly, normal spermatic vessels entered in the internal closed inguinal ring, bilaterally. Histology revealed residual fallopian tubes alongside a rudimental uterus with no recognizable deferential vases reaching the uterine wall and or fusing in it.
The patient has no hormonal alteration and bilateral healthy testes in the scrotum at twelve years old follow up.
Results and discussion
In Persistent Mullerian Duct Syndrome (PMDS) testes are normally differentiated and, in absence of longstanding cryptorchidism, usually contain functional germ cells. However, it is commonly associated with high percentage of infertility due to aplasia/hypoplasia of the epididymis and/or upper part of deferens, and to narrow, blind or even absent vases, too [1]. Typically, the vas deferens is included in the mesosalpinx, then reaches the uterine wall and eventually penetrating it to open at the top of vagina, the anatomical equivalent of the prostatic utricle. So any attempt to remove the mullerian organs will automatically damage the vas for their strict relationship [2,3]. Accordingly, it is recommended to not dissected the fimbriae of the fallopian tube from the testes and to left adhering the myometrium to the vas, if the spermatic vessels are too short and a Fowler- Stephen two-stage procedure is indicated [4,5]. In fact, this technique might guarantee the vascular supply of the testis through vas artery. However, for several reasons, leaving the Müllerian remnants in place is not advocated. Actually, it seems there might be an intrinsic tendency of Mullerian ducts to manifest into cancers and/or to cause haematuria to response of excess of estrogenic hormone synthesis in aging patient [6]. Therefore, if intra-abdominal testes with their cords present a high degree of mobility, the authors suggest one stage orchidopexy. This could be carry out one by one, before the 18 months of life for two reasons: 1) A major possibility to reach the root of scrotum due to the real shortness of the inguinal canal; 2) To reduce the malignant degeneration to the well-known longstanding cryptorchidism. Likewise, a complete removal of mullerian ducts is otherwise supported. Besides, since a testicular malignancy is reported to occurs after puberty in 33% of cases, despite an early orchidopexy, a long- term follow up is mandatory [7]. Regard the fertility issue, assuming the presence of a normal testicular tissue pattern, the testicular sperm extraction followed by intracytoplasmic sperm injection technique, could be considered to treat abnormalities in absence of male excretory ducts, whether congenital or iatrogenic. This procedure has been reported in several case since 1994, giving to PMDS patients a slight hoping of fatherhood [8].
Conclusion
In Persistent Mullerian Duct Syndrome (PMDS) with bilateral cryptorchidism, a surgical treatment of three stage procedures is recommended to rescue testes and pull them down healthy in the scrotum. If a high degree of testis mobility is observed, one stage orchidopexy on spermatic vessels has to be considered. This should be carry out within 18 months of age for the shortness of inguinal canal and the increased risk of cancer in longstanding cryptorchidism. Furthermore, it should be always considered if a complete resection of mullerian ducts is advocated, due to the elevated rescue of vas injury during the removal procedure.
Author Consent
Informed consent was taken from the patient.
References
- Picard JY, Cate RL, Racine C, et al. The persistent müllerian duct syndrome: An update based upon a personal experience of 157 cases. Sex Dev. 2017;11(3):109-125.
[Crossref] [Google Scholar] [PubMed]
- Gutte AA, Pendharkar PS, Sorte SZ. Transverse testicular ectopia associated with persistent mullerian duct syndrome-the role of imaging. Br J Radiol. 2008;81(967):e176-e178.
[Crossref] [Google Scholar] [PubMed]
- Alanazi AB, Aldhowayan A, Almuhanna MM, et al. Persistent Mullerian Duct Syndrome (PMDS): Case report and review of literature. Urol Case Rep. 2022;42:102031.
[Crossref] [Google Scholar] [PubMed]
- Wongprasert H, Somanunt S, De Filippo R, et al. A novel mutation of anti-mullerian hormone gene in persistent mullerian duct syndrome presented with bilateral cryptorchidism: A case report. J Pediatr Urol. 2013;9(4):e147-e149.
[Crossref] [Google Scholar] [PubMed]
- Josso N, Belville C, Di Clemente N, et al. AMH and AMH receptor defects in persistent Müllerian duct syndrome. Hum Reprod Update. 2005;11(4):351-356.
[Crossref] [Google Scholar] [PubMed]
- Manjunath BG, Shenoy VG, Raj P. Persistent müllerian duct syndrome: How to deal with the müllerian duct remnantsâ?a review. Indian J Surg. 2010;72:16-19.
[Crossref] [Google Scholar] [PubMed]
- Farikullah J, Ehtisham S, Nappo S, et al. Persistent Müllerian duct syndrome: Lessons learned from managing a series of eight patients over a 10 year period and review of literature regarding malignant risk from the müllerian remnants. BJU Int. 2012;(110):e1084-e1089.
[Crossref] [Google Scholar] [PubMed]
- Popal W, Nagy ZP. Laboratory processing and intracytoplasmic sperm injection using epididymal and testicular spermatozoa: What can be done to improve outcomes?. Clinics. 2013;68:125-130.
[Crossref] [Google Scholar] [PubMed]
Author Info
Germana Casaccia1*, Lorenzo Giacometti2 and Andrea Secco32Department of Pathology, OU SS Antonio e Biagio e Cesare Arrigo, Spalto Marengo, Alessandria, 46, 15, Italy
3Department of Pediatrics , The Children Hospital “Cesare Arrigo”, Spalto Marengo, Alessandria, 46, 1, Italy
Received: 19-Aug-2024, Manuscript No. PUCR-24- 145754; , Pre QC No. PUCR-24-145754 (PQ); Editor assigned: 21-Aug-2024, Pre QC No. PUCR-24-145754 (PQ); Reviewed: 04-Sep-2024, QC No. PUCR-24- 145754; Revised: 11-Sep-2024, Manuscript No. PUCR-24-145754 (R); Published: 18-Sep-2024, DOI: 10.14534/j-pucr.20222675676
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.