Case Report - (2025) Volume 12, Issue 6
Survival with Bilateral Multicystic Dysplastic Kidneys: A case report
Paddy Dewan1*, Padma Rao2, Mandar Massada da Rocha2, Renata Dhirajla1 and Adalberto Clemente Boca2Abstract
The multicystic dysplastic kidney is now understood to be due to the combination of a vascular accident to both the ureteric bud and the renal blastema resulting in a characteristic appearance, but with wide variation possible. We present the case of a girl with evidence of the prenatal loss of blood supply of her only functioning kidney, which may be the only such case recorded, as the other two cases recorded may well not have the same aetiology because the morphology appeared to differ from the asserted embryop athy. The surgery required in our case differed from a normal pyeloplasty (for the pelviureteric junction ob struction component of the uropathy) to ensure the remaining blood supply to the minimal parenchyma was not compromised.
Keywords
Multicystic dysplastic kidney, Ureteric atresia, Obstruction, Aberrant vessels, Pyeloplasty
Introduction
A Multicystic Dysplastic Kidney (MCDK) often has a unique “Bunch of Grapes” appearance, first described by Cruveilhier J, et al., in 1836 and further studied by Schwartz J in 1936 [1,2]. It is now known to have various appearances depending on the stage of involution after the vascular accident that causes the changes outlined in the Dewan PA, et al., study in 1991 [3]. Their study showed that the cysts interconnect and supports the view that the ureteric bud connects to the renal blastema followed by a vascular accident that results in the cystic changes that relate to the ischaemia and concurrent ureteric atresia. Despite the long history since the initial recognition of the condition, the pathogenesis of the MCDK has still not been resolved and there is often confusion between cystic renal dysplasia and MCDK, the latter being differentiated by the presence of ureteric atresia or stenosis. The Potter classification adds to this confusion by having a case of urethral obstruction illustrated as a Type IIB kidney, the group that most closely resembles the MCDK [4]. The coexistence of the ureteric and renal abnormalities suggests an partly obstructive aetiology [5,6], particularly as there is commonly a pelviureteric junction abnormality on the contralateral side [7]. However, the ureter is not always completely obstructed, and the upper ureter and pelvis can be dilated above a more distal obstruction [5,8], as was demonstrated in the ex-vivo radiographs in the Dewan PA, et al., paper [3].
Survival of patients with bilateral MCDK is unexpected, thus the importance of reporting this case.
Case Presentation
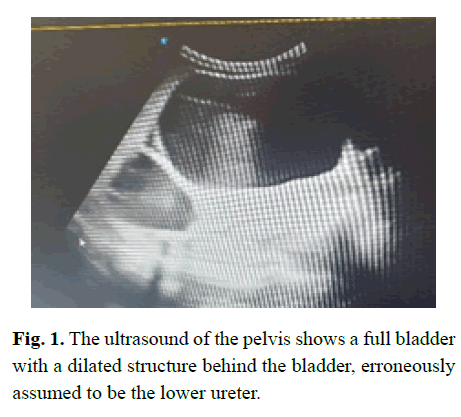
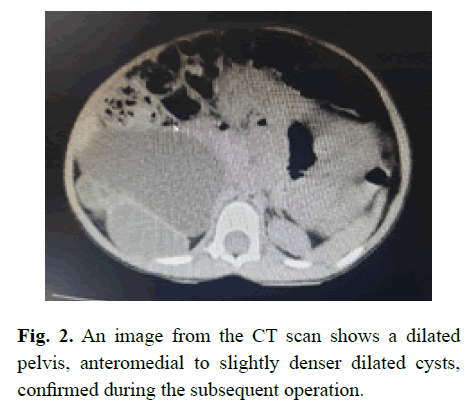
A 3-year-old old girl presented with an abdominal mass, and was found on investigation in Mozambique to have a small cystic kidney on the left and a dilated ureter below a dilated pelvis of an abnormal kidney on the right; a kidney that appeared to contain cystic areas on both ultrasound (Figure 1) and CT scanning (Figure 2); and the dilated structure in the pelvis that was incorrectly assumed to be the ureter being dilated to the entry into the bladder.
Figure 1: The ultrasound of the pelvis shows a full bladder with a dilated structure behind the bladder, erroneously assumed to be the lower ureter.
Figure 2: An image from the CT scan shows a dilated pelvis, anteromedial to slightly denser dilated cysts, confirmed during the subsequent operation.
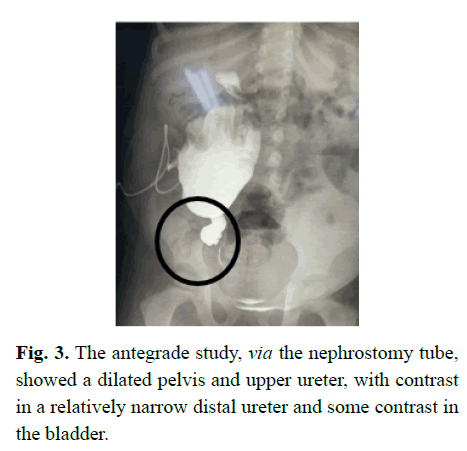
The uncertainty of her anatomy led to the placement of a nephrostomy tube, undertaken to both further evaluate her urological anatomy, and review her renal function, via assessment of her urine quality. Erect (Figure 3) and supine images demonstrated an obstruction in the ureter and a markedly dilated pelvis; and suggested a normal bladder.
Figure 3: The antegrade study, via the nephrostomy tube, showed a dilated pelvis and upper ureter, with contrast in a relatively narrow distal ureter and some contrast in the bladder.
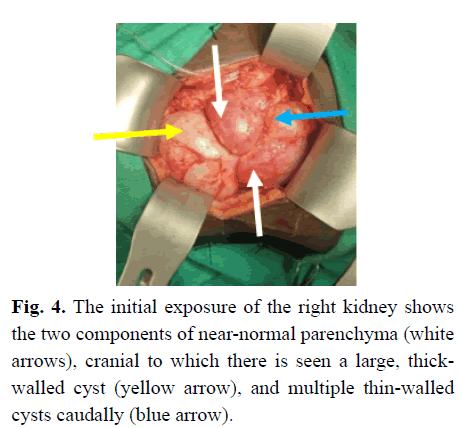
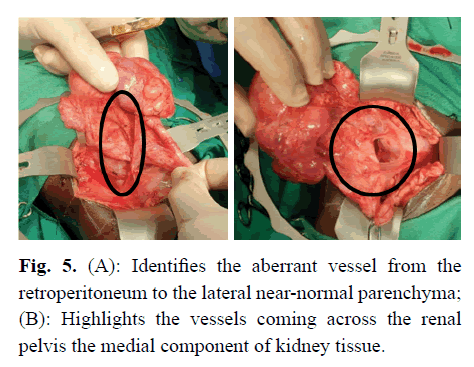
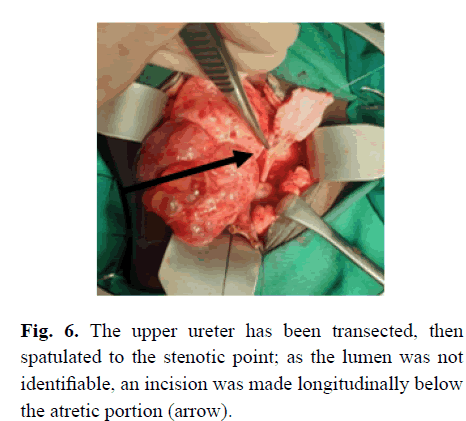
Subsequently, at operation, she was found to have a forme fruste MCDK. There appeared to be two near-normal portions of kidney, one served by the aberrant extraperitoneal vessel and the other medial to that segment. A large thick-walled cyst was cranial and partly between the renal parenchyma, with a cystic area caudally and multiple smaller cysts cranial to the large cyst (Figure 4). The upper ureter, above the atretic portion was dilated and it was noted that there was an abnormal blood supply coming from the retroperitoneum caudal and lateral to the kidney (Figure 5A), and via additional blood vessels coming across the pelvis anteromedially (Figure 5B). She also had near-complete ureteric atresia, with the orifice not being able to be cannulated from above with a 22-gauge intravenous cannula. Thus, the ureter distal to the obstruction was opened to identify the lumen (Figure 6), after which a dismemberment pyeloplasty was performed, but with no resection of the pelvis because of the apparent reliance of the renal tissue on the blood supply via the renal pelvic tissue.
Figure 4: The initial exposure of the right kidney shows the two components of near-normal parenchyma (white arrows), cranial to which there is seen a large, thickwalled cyst (yellow arrow), and multiple thin-walled cysts caudally (blue arrow).
Figure 5:(A): Identifies the aberrant vessel from the retroperitoneum to the lateral near-normal parenchyma; (B): Highlights the vessels coming across the renal pelvis the medial component of kidney tissue.
Figure 6: The upper ureter has been transected, then spatulated to the stenotic point; as the lumen was not identifiable, an incision was made longitudinally below the atretic portion (arrow).
Initially the creatinine was 140, which fell to 122 after the insertion of a nephrostomy tube, but after the pyeloplasty the renal function deteriorated, transiently, with a level of around 200, associated with heavy haematuria. Despite passing urine from her bladder, when the nephrostomy catheter was clamped, the creatinine again became significantly elevated to 400, and reduced to 200 with the catheter on drainage. Since the removal of the nephrostomy tube that level has stabilized at 100. Unfortunately, the intra-operative insertion of a double J stent was not possible due to resource limitation in Mozambique.
Discussion
Bilateral MCDK would be expected to be a nonsurvivable pathology but there have been two previous case reports, both in 2025 suggesting survival. On review of the imaging in those studies, the Tanzanian case may not be a classic MCDK [9] and, although the Iranian case describes multiple cysts in both kidneys, the cystogram appears more like vesicoureteric reflux disease, and the child also had a pyeloplasty on the right side.
The detail of the description did not confirm the anatomy consistent with a vascular accident to the ureter and renal blastema that is strongly evident in our case [10]. However, supporting the prospect of survivability, the Dewan PA, et al., study, in which 11 excised kidneys were investigated with a MAG III nuclear medicine study prior to removal showed minimal but definite function in 6 cases [3], supporting the interpretation that the renal parenchyma may develop alternative blood supply and survive, as is shown clearly in our presented case. We know the MCDK is the commonest cause of a renal mass in the newborn [11], and was first described in 1836 [1], with prenatal ultrasound now having demonstrated the evolution of the cystic changes [12,13].
Prior to the study by Dewan PA, who showed function in 6 of 11 MCDK kidneys, and cyst communication in 10 of 11 cases, it was widely considered that MCDKs had no function [14,15] and their cysts did not communicate [8,16-21]. However, the in vivo radiographic studies of Saxton HM, et al., and Kullendorff CM, et al., and microdissections of Madewell JE, et al., also demonstrated the interconnecting tubules [19-22].
The communication between the cysts indicates that there has been a connection between the ureteric bud and the renal blastema, precluding a primary defect of the ureteric bud. A purely vascular accident mechanism has been proposed; this is supported by the presence of a rudimentary vascular pedicle in virtually all cases, [23,24] and the common association with crossed renal ectopia [25,26], duplex systems [27] and horseshoe kidneys [7,28], where variations of the renal vessel anatomy are always present. However, the features of the MCDK seem to be both loss of blood supply to both parenchyma and ureter, suggested by the change from a minimally hydronephrotic kidney in utero to a classic MCDK, as observed by Avni EF, et al [13].
Their finding suggests that the phenomenon is similar to the vascular accident seen with small and large bowel atresia [29,30]. Fetal studies of the model would suggest that the anomalies are due to a failure of apoptosis around the ureteric bud and renal blastema—the link may be the inability of blood vessels to grow through the ‘rind’ of cells at the pelviureteric junction; thus ischaemia plus obstruction occurs [31]. Dewan PA and Goh DW showed that the degree of ureteric stenosis is more variable than previously understood, showing, in an otherwise typical MCDK [3], a dilated pelvis and upper ureter. A radiographically patent ureter [8,32] or complete absence of the ureter have also been observed by others [8]. If the aetiology is obstruction alone, one would expect complete occlusion in all cases. And, animal models of ureteric obstruction alone do not produce the “Bunch of Grapes” appearance of the MCDK [33].
Conclusion
Multicystic dysplastic kidneys are now well understood to be a vascular accident to the developing renal blastema and ureteric bud. Our case may be the first definitive case of survival from bilateral disease, and highlights the need to careful surgery for the obstructive component to ensure that all potential blood supply is preserved.
References
- Cruveilhier J, Chazal A. Anatomie pathologique. JB Baillière. 1836.
- Schwartz J. An unusual unilateral multicystic kidney in an infant. J Urol. 1936:35:259-263.
- Dewan PA, Goh DW. A study of the radiological anatomy of the multicystic kidney. Pediatr Surg Int. 1994;9(5):368-372.
- Potter EL. Normal and abnormal development of the kidney. Chicago: Year Book Med Pub Inc. 1972:154-181.
- Sumner T, Friedland GW, Parker B, et al. Preoperative diagnosis of unilateral multicystic kidney with hydropelvis. Urology. 1978;11(5):519-522.
[Crossref] [Google Scholar] [PubMed]
- Duckett JW, Filmer RB. Another variant of the multicystic kidney. J Pediatr Surg. 1976:11:93-94.
[Crossref] [Google Scholar] [PubMed]
- Greene LF, Feinzaig W, Dahlin DC. Multicystic dysplasia of the kidney: With special reference to the contralateral kidney. J Urol. 1971:105:482-487.
[Crossref] [Google Scholar] [PubMed]
- Griscom NT, Vawter GF, Fellers FX. Pelvifundibular atresia: The usual form of multicystic kidney: 44 unilateral and two bilateral cases. Semin Roentgenol. 1975:10:125-131.
[Crossref] [Google Scholar] [PubMed]
- Chaki S, Mclarty R, Mzolo I, et al. A rare case of antenatal bilateral multicystic dysplastic kidney disease: An unusual presentation in a neonate. Clin Case Rep. 2025;13(4):e70407.
[Crossref] [Google Scholar] [PubMed]
- Pourpashang P, Tabatabaei SMTH, Fallahi M, et al. An infant girl with bilateral multicystic dysplastic kidney: A case report. J Nephropathol. 2025;14(1):e21432.
- Raffensperger J, Abousleiman A. Abdominal masses in children under on year of age. Surgery. 1968;63(3):514-521.
[Google Scholar] [PubMed]
- Hashimoto BE, Filly RA, Callen PW. Multicystic dysplastic kidney in utero: Changing appearance on US. Radiology. 1986;159(1):107-109.
[Crossref] [Google Scholar] [PubMed]
- Avni EF, Thoua Y, Lalmand B, et al. Multicystic dysplastic kidney: Natural history from in utero diagnosis and postnatal followup. J Urol. 1987;138(6):1420-1424.
[Crossref] [Google Scholar] [PubMed]
- Thomas DF, Najmaldin AS. Multicystic dysplastic kidney. Newborn Surg. 2017:1048-1056.
- Warshawsky AB, Miller KE, Kaplan GW. Urographic visualization of multicystic kidneys. J Urol. 1977;117(1):94-96.
[Crossref] [Google Scholar] [PubMed]
- Wood BP, Goske M, Rabinowitz R. Multicystic renal dysplasia masquerading as ureteropelvic junction obstruction. J Urol. 1984;132(5):972-974.
[Crossref] [Google Scholar] [PubMed]
- Carey PO, Howards SS. Multicystxc dysplastic kidneys and diagnostic confusion on renal scan. J Urol. 1988;139(1):83-84.
[Crossref] [Google Scholar] [PubMed]
- Felson B, Cussen LJ. The hydronephrotic type of unilateral congenital multicystic disease of the kidney. Semin Roentgenol. 1975;10(2):113-123.
[Crossref] [Google Scholar] [PubMed]
- Saxton HM, Golding SJ, Chantler C, et al. Diagnostic puncture in renal cystic dysplasia (multicystic kidney). Evidence on the aetiology of the cysts. Br J Radiol. 1981;54(643):555-561.
[Crossref] [Google Scholar] [PubMed]
- Kullendorff CM, Salmonson EC, Laurin S. Diagnostic cyst puncture of multicystic kidney in neonates. Acta Radiol. 1990;31(3):287-289.
[Google Scholar] [PubMed]
- Peters CA, Mandell J. The multicystic dysplastic kidney. AUA Update Series. 1989;7:50-55.
- Madewell JE, Hartman DS, Lichenstein JE. Radiologic-pathologic correlation in cystic disease of the kidney. Radiol Clin North Am. 1979;17:261-279.
[Google Scholar] [PubMed]
- Ljungqvist A. Arterial vasculature of the multicystic dysplastic kidney. A micro-angiographical and histological study. Acta Pathol Microbiol Scand. 1965;64:309-317.
[Crossref] [Google Scholar] [PubMed]
- Greenberg LA, Altman DH, Litt RE. Cystic enlargement of the kidney in infancy. Radiology. 1967;89:850-856.
[Crossref] [Google Scholar] [PubMed]
- Herczeg T, Rutkai P, Deák J. Metanephrogenic cystic remnant of crossed dystopic kidney. J Urol. 1956;76:488-498.
[Crossref] [Google Scholar] [PubMed]
- Nussbaum AR, Hartman DS, Whitley N, et al. Multicystic dysplasia and crossed renal ectopia. AJR Am J Roentgenol. 1987;149(2):407-410.
[Crossref] [Google Scholar] [PubMed]
- Diard F, Le Dosseur P, Cadier L, et al. Multicystic dysplasia in the upper component of the complete duplex kidney. Pediatr Radiol. 1984;14:310-313.
[Crossref] [Google Scholar] [PubMed]
- Novak ME, Baum NH, Gonzales Jr ET. Horseshoe kidney with multicystic dysplasia associated with ureterocele. Urology. 1977;10(5):456-458.
[Crossref] [Google Scholar] [PubMed]
- Louw JH. Jejunoileal atresia and stenosis. J Pediatr Surg. 1966;1:8-23.
- Dewan PA. Colonic atresia. Newborn Surgery. Oxford: Butterworth-Heinemann. 1991:318-323.
- Yerkes E, Nishimura H, Hunley T, et al. Association between the Angiotensin Type 2 receptor (AT2) genotype and congenital urinary tract anomalies in mice and men. Pediatric Res. 1997;41(4):286.
- Uehling D, Barber KE. Unilateral multicystic kidney. J Urol. 1966;96:286-289.
[Crossref] [Google Scholar] [PubMed]
- Beck AD. The effect of intra-uterine urinary obstruction upon the development of the fetal kidney. J Urol. 1971;105:784-789.
[Crossref] [Google Scholar] [PubMed]
Author Info
Paddy Dewan1*, Padma Rao2, Mandar Massada da Rocha2, Renata Dhirajla1 and Adalberto Clemente Boca22Department of Medical Imaging, Royal Children’s Hospital, Melbourne, Vic, Australia
Received: 29-Aug-2025, Manuscript No. PUCR-25-170511; , Pre QC No. PUCR-25-170511 (PQ); Editor assigned: 01-Sep-2025, Pre QC No. PUCR-25-170511 (PQ); Reviewed: 29-Sep-2025, QC No. PUCR-25-170511; Revised: 06-Oct-2025, Manuscript No. PUCR-25-170511 (R); Published: 13-Oct-2025, DOI: 10.14534/j-pucr.20222675714
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.